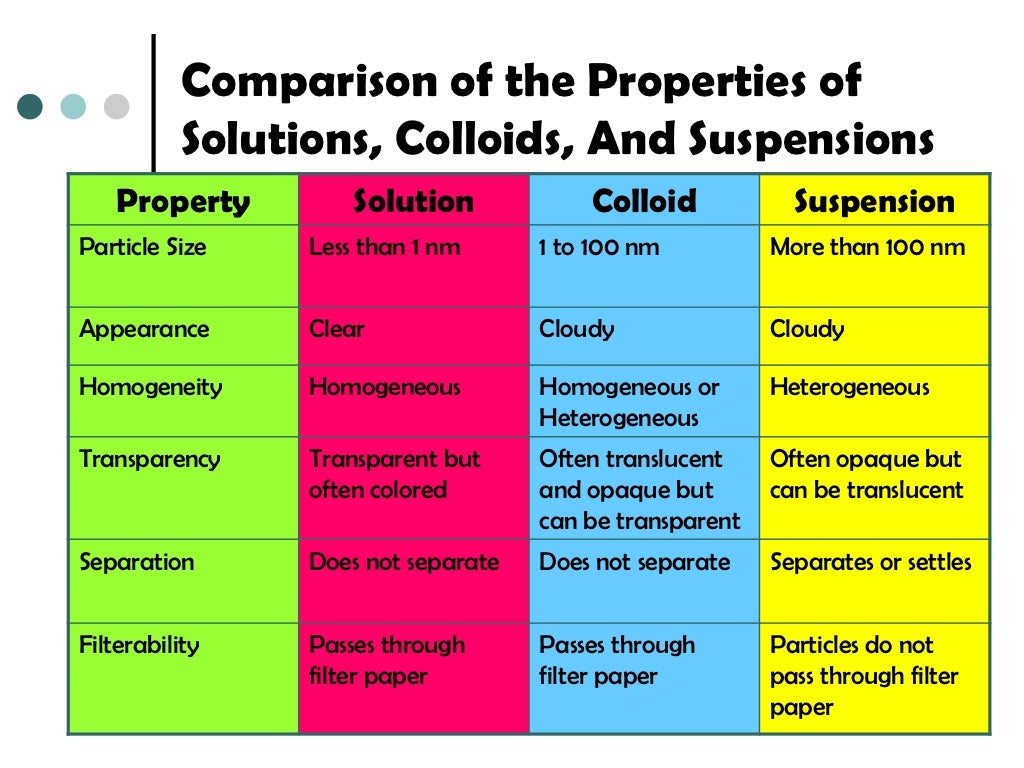
Solution Vs Colloid Difference . You will also learn the. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. For example, kcl(s) + h 2o →. The particle size in a solution is smaller than in a colloid,. the main difference between a solution and a colloid lies in the particle size and homogeneity of the mixture. this post describes the key differences between colloid and solution, along with the comparison chart. difference between a colloid and a solution. Colloids consist of two distinct phases: Also, the solute and solvent constitute one. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. Here are the key differences:. a solution consists of a single phase whereby a solute is solvated by a solvent. a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. A colloid is a homogeneous.
from cegexkfz.blob.core.windows.net
this post describes the key differences between colloid and solution, along with the comparison chart. a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. Also, the solute and solvent constitute one. Here are the key differences:. For example, kcl(s) + h 2o →. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. You will also learn the. a solution consists of a single phase whereby a solute is solvated by a solvent. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Colloids consist of two distinct phases:
Colloid Solutions Quizlet at Katrina Cohen blog
Solution Vs Colloid Difference A colloid is a homogeneous. For example, kcl(s) + h 2o →. a solution consists of a single phase whereby a solute is solvated by a solvent. a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. the main difference between a solution and a colloid lies in the particle size and homogeneity of the mixture. Here are the key differences:. difference between a colloid and a solution. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. The particle size in a solution is smaller than in a colloid,. Also, the solute and solvent constitute one. Colloids consist of two distinct phases: this post describes the key differences between colloid and solution, along with the comparison chart. You will also learn the. A colloid is a homogeneous.
From ar.inspiredpencil.com
Colloids Suspensions And Solutions Solution Vs Colloid Difference A colloid is a homogeneous. difference between a colloid and a solution. Colloids consist of two distinct phases: a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. a solution consists of a single phase whereby a solute is solvated by a solvent. The particle size in a. Solution Vs Colloid Difference.
From captionsfeaturenl.blogspot.com
what is the difference between a solution and a suspension Captions Solution Vs Colloid Difference in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Colloids consist of two distinct phases: difference between a colloid and a solution. the main difference between a solution and a colloid lies in the particle size and homogeneity of the mixture. The particle size. Solution Vs Colloid Difference.
From cegexkfz.blob.core.windows.net
Colloid Solutions Quizlet at Katrina Cohen blog Solution Vs Colloid Difference the main difference between a solution and a colloid lies in the particle size and homogeneity of the mixture. The particle size in a solution is smaller than in a colloid,. You will also learn the. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. a colloid results when particles ranging between 1 and. Solution Vs Colloid Difference.
From brainly.in
what is the different between solution suspension and colloid Brainly.in Solution Vs Colloid Difference A colloid is a homogeneous. a solution consists of a single phase whereby a solute is solvated by a solvent. a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. You will also learn the. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. For. Solution Vs Colloid Difference.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Solution Vs Colloid Difference Colloids consist of two distinct phases: Here are the key differences:. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. The particle size in a solution is smaller than in a colloid,. You will also learn the. the main difference between a solution and a colloid lies in the particle size and homogeneity of the. Solution Vs Colloid Difference.
From ar.inspiredpencil.com
Suspensions Mixtures Solution Vs Colloid Difference You will also learn the. a solution consists of a single phase whereby a solute is solvated by a solvent. this post describes the key differences between colloid and solution, along with the comparison chart. A colloid is a homogeneous. the main difference between a solution and a colloid lies in the particle size and homogeneity of. Solution Vs Colloid Difference.
From courses.lumenlearning.com
Colloids Chemistry for Majors Solution Vs Colloid Difference For example, kcl(s) + h 2o →. a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. Also, the solute and solvent constitute one. You will also learn the. difference between a colloid and a solution. in this chapter, we will consider the nature of solutions, and examine. Solution Vs Colloid Difference.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Solution Vs Colloid Difference You will also learn the. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. this post describes the key differences between colloid and solution, along with the comparison chart. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Also, the solute. Solution Vs Colloid Difference.
From www.youtube.com
SOL vs GEL Fast differences and comparison YouTube Solution Vs Colloid Difference A colloid is a homogeneous. difference between a colloid and a solution. Here are the key differences:. For example, kcl(s) + h 2o →. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. a solution consists of a single phase whereby a solute is. Solution Vs Colloid Difference.
From exobkcoth.blob.core.windows.net
Definition Of Suspension In Chemistry Class 9 at Jeff Obryan blog Solution Vs Colloid Difference a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. You will also learn the. difference between a colloid and a solution. A colloid is a. Solution Vs Colloid Difference.
From www.youtube.com
Mixtures and solutions venn diagram YouTube Solution Vs Colloid Difference a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. this post describes the key differences between colloid and solution, along with the comparison chart. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. . Solution Vs Colloid Difference.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Solution Vs Colloid Difference a solution consists of a single phase whereby a solute is solvated by a solvent. difference between a colloid and a solution. Here are the key differences:. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Also, the solute and solvent constitute one. You. Solution Vs Colloid Difference.
From www.freepik.com
Premium Vector Faraday tyndall effect vector illustration Solution Vs Colloid Difference difference between a colloid and a solution. The particle size in a solution is smaller than in a colloid,. For example, kcl(s) + h 2o →. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. this post describes the key differences between colloid and solution, along with the comparison chart. Here are the key. Solution Vs Colloid Difference.
From scsscience9.blogspot.com
Is Matter Around Us Pure? Blog 6 Solution Vs Colloid Difference A colloid is a homogeneous. Here are the key differences:. this post describes the key differences between colloid and solution, along with the comparison chart. The particle size in a solution is smaller than in a colloid,. Also, the solute and solvent constitute one. For example, kcl(s) + h 2o →. You will also learn the. a colloid. Solution Vs Colloid Difference.
From cekjtznh.blob.core.windows.net
Colloid Compound Solution Suspension at Robert Rutledge blog Solution Vs Colloid Difference The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. A colloid is a homogeneous. Here are the key differences:. this post describes the key differences between colloid and solution, along with the comparison chart. For example, kcl(s) + h 2o →. a solution consists of a single phase whereby a solute is solvated by. Solution Vs Colloid Difference.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Solution Vs Colloid Difference a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. For example, kcl(s) + h 2o →. Colloids consist of two distinct phases: The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving. the main difference between a solution and a colloid lies in the particle. Solution Vs Colloid Difference.
From exouqcyfe.blob.core.windows.net
What Is Colloidal Suspension In Chemistry at Robert Lemond blog Solution Vs Colloid Difference a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. For example, kcl(s) + h 2o →. The particle size in a solution is smaller than in a colloid,. You will also learn the. Colloids consist of two distinct phases: The dispersed phase (particles) and the continuous phase (medium), which. Solution Vs Colloid Difference.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Solution Vs Colloid Difference difference between a colloid and a solution. a colloid results when particles ranging between 1 and 1000 nanometers in diameter are dispersed in the liquid solvent. the main difference between a solution and a colloid lies in the particle size and homogeneity of the mixture. a solution consists of a single phase whereby a solute is. Solution Vs Colloid Difference.